Empowering
Clinical
Research
Together
Enabling Life-Changing Therapies with Excellence and Commitment
ABOUT US
Global MedTrial
Since 2010, Medtrial Solutions (Global MedTrial) CRC team has been successfully providing services in many research sites of different clinical trial projects, in areas such as medical devices, chemical and biological products. Our therapeutic experience includes oncology, cardiovascular, anti-infection, autoimmune diseases, endocrine system disease, neural diseases, respiratory diseases, ophthalmology and digestive diseases. We focus on the following diseases: lung cancer, liver cancer, gastric cancer, Alzheimer’s disease, schizophrenia, epilepsy and diabetes mellitus. We have experience in clinical trials from Phase I to Phase IV, non-interventional outcome studies and medical device studies.

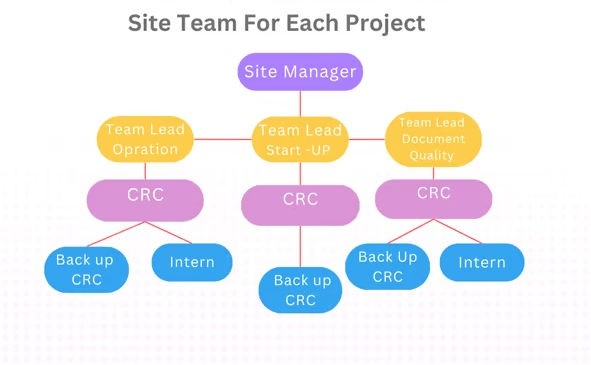
Experienced team, efficient management processes
- Global MedTrial has own SITE SOPs and a clinical research coordinator (CRC) management team with extensive clinical trial experience. Our managers and Team Leaders have years of experience in site management, overseas clinical trials and clinical operations at global pharmaceutical companies. We fully understand the demands of sponsors.
All members of our highly qualified CRC team hold a Bachelor’s or equivalent degree and higher qualifications in nursing, medicine, pharmaceutical sciences , Life Scienceor other related medical sciences,. Most team members have worked in hospitals and are familiar with the work processes in the hospital environment,
Looking to streamline your clinical research?
Partner with Global MedTrial for expert support in clinical trials from Phase I to IV, medical device studies, and non-interventional research. Our experienced CRC team is ready to assist you across multiple therapeutic areas.
Experienced team, efficient management processes
Global MedTrials Solutions has own SITE SOPs and a clinical research coordinator (CRC) management team with extensive clinical trial experience. Our managers and Team Leaders have years of experience in site management, overseas clinical trials and clinical operations at global pharmaceutical companies. We fully understand the demands of sponsors.
All members of our highly qualified CRC team hold a Bachelor’s or equivalent degree and higher qualifications in nursing, medicine, pharmaceutical sciences , Life Scienceor other related medical sciences,. Most team members have worked in hospitals and are familiar with the work processes in the hospital environment,